Latest news about Trauma and Orthopaedics:
08.06.16
IT issues in T&O
At the 2016 annual general meeting Sunny Deo (Swindon) showed the results of a very comprehensive survey on the frustrations caused in orthopaedic departments by poor IT.
His powerpoints can be downloaded below.
The work raises remarkably similar issues to those identified in a very comprehensive survey on professional satisfaction which was carried out by the Rand Corporation for the American Medical Association. This interesting document can also be downloaded below.
08.06.16
BODS Annual General Meeting 2016
The 2016 annual general meeting was held at the Royal Orthopaedic Hospital, Birmingham. The meeting was very well attended with around 30 clinical directors who shared their experiences and listened to a number of useful presentations.
Ro Kulkarni started proceedings by giving a very detailed talk on the intricacies of the tariff system. Scott Muller then told us about the reorganisation of trauma around a specialist emergency hospital in Northumbria. There was then a seminar on preparation for clinical leadership given by Lisa Hadfield-law, Phil Turner, And Hiro Tanaka.
There was then an excellent free lunch. Andrew Thomas had organised for the higher surgical training programme to have a clinical meeting elsewhere and the Knowledge Hub staff had very helpfully not cancelled the order for the trainees lunch!
Tim Wilton, BOA president, then talked about the practicalities of GiRFT implementation. His talk was illustrated by screenshots of the dashboard for his own hospital. There was discussion about various bugs in the system and Tim will be giving useful feedback to the implementation team.
Gray Giddings then spoke on MSK networks and did a remarkable job of making the subject entertaining. Another talk from the north-east was given by Rob Gregory who discussed the development of an MSK network in Northumbria.
At the annual general meeting Sudhi Ankarath handed over the presidency of BODS to Vinay Takwale.
21.05.15
BODS Annual General Meeting 2015
A very successful annual general meeting was held on Friday 8th May 2015 in Cheltenham. The local host was Vinay Takwale.
The guest speakers were Peter Kay, national clinical director for musculoskeletal services, Tim Briggs, who spoke on the future of the GIRFT project and the chief executive of the Cheltenham and Gloucester NHS trust who gave a fascinating talk on his approach to leadership in his organisation.
Karl Trimble from Plymouth spoke on his experience with commissioning and Andrew Thomas gave details of a BOA project to look at the outcomes of different methods of orthopaedic triage. Paul Carter from Aintree spoke on a ring fenced orthopaedic beds project and Ro Kulkarni from Royal Gwent spoke on virtual fracture clinics.
Jeremy Ridge concluded by giving an entertaining talk on the history of BODS, in the best traditions of orthopaedic accounts of the good old days.
At the AGM it was agreed that the organisation should have a secretary, who would be the president elect. Vinay Takwale Volunteered for this post and, in view of his good local organisation, he received unanimous support.
Andrew Thomas asked for a volunteer to help maintain the website and Ajit Shetty has agreed to help.
There will be shortly a vacancy to represent BODS on the BOA professional practice committee, anyone interested should contact Sudhi Ankarath.
21.05.15
Downloads from the annual general meeting:
08.12.14
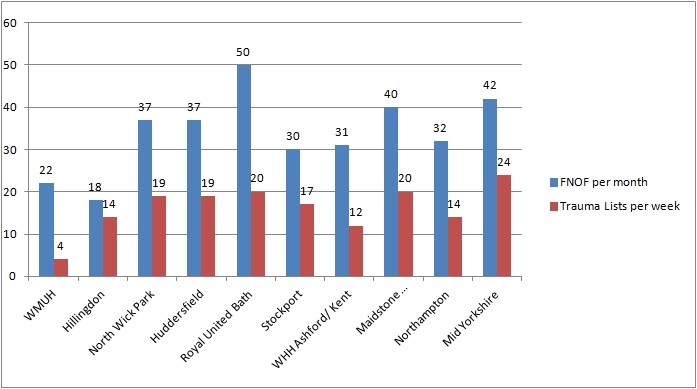
Trauma Lists
A survey was carried out by Soosai Nathan on trauma list provision. The findings are condensed into a graph showing the responses. It is possible to review the rates of trauma theatre provision related to the number of fractured necks of femur that a unit admits. CDs will be able to see if their provision hits the going rate. Trusts and patients will benefit if the trust hits best practice tarrif.
09.10.2014
European union medical device approvals legislation.
The following information appeared in the recent EFORT newsletter and it may well have relevance for new developments in trauma and orthopaedics:
The European Union may be moving toward stricter medical device regulations following an implementing act issued in September 2013 by the European Commission. New legislation has been proposed to make approval laws for medical devices the same in all European Union regulatory bodies, and if approved, it will take effect in 2017. If approved, the new uniform approval process for medical devices will also add time, possibly multiple years, to the approval of a new product. If the approvals process becomes longer and more complicated, it is bound to increase the cost of development. It is probably not going to make medical devices any cheaper in Europe.
Currently, to obtain a European CE mark or Conformity Assessment for a new medical device, a company submits an application to one of 75 EU Notified Bodies, a certification organisation designated by the national authority of a member state. The Notified Body will see if a medical device company’s application for a new product is approved by a Conformity Assessment, which examines a potential device’s design, full quality assurance, product quality assurance and other qualifications. There is no requirement to prove the efficacy of the product and only basic safety requirements. If the Declaration of Conformity is granted by one notified body to a medical device, it could be sold throughout the entire European community.
The self-certification nature of the current process means it is quick for manufacturers to get a device onto the market, however, according to John Wilkinson, of the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom, “Many of us thought that this was an unsatisfactory situation because it leaves a lot of room for different interpretations of the rules. Potentially, it mean that Notified Bodies in different countries were operating with slightly different standards.” If the new legislation is approved by the European Parliament, Notified Bodies would go through a full re-designation process by a joint audit including the European Parliament, the competent authorities of the member state and two other member states to bring all Notified Bodies under the same set of rules. “The end result will be, we hope, a much more consistent performance both by member states and the Notified Bodies. The idea is to bring this into one system operating consistently via a very clear set of rules”
BRITISH ORTHOPAEDIC DIRECTORS SOCIETY BOA REPORT
JUNE 2015
Annual meeting:
BODS held its annual meeting in May in Cheltenham. The low attendance did not reduce the quality of discussions on wide ranging topics, with excellent talks from various speakers including Peter Kay and Tim Briggs.
Summary of BODS web forum discussions:
Follow-ups in uncomplicated Primary THRs and TKRs:
Even among a small sample of 11 units, there was considerable variation in practice on routine follow up of primary uncomplicated THR and TKR patients. Most units bring patients back at 6 weeks and 1 year to consultant led clinics. Some see patients at 3 months and a minority have nurse follow-ups at 1 year before discharge.
Use of Hoods for TJRs:
Of the 13 responses, 4 did not routinely use hoods while performing total joint replacements. There was an acknowledgment that this was more for personal protection of the scrub members and adds little to reducing infection risks any further when combined with other established infection control practices such as antibiotic prophylaxis, clean air theatre etc..
Chloraprep:
It was noted that some Trusts were forcing clinicians to use Chloraprep (2% Chlorhexidine) for preop. skin preparation as this was the only licensed product in the market, on the back of MHRA and RCS recommendations. It was felt that the unreasonable cost of the product was not justified. The members raised concerns that the benefits in using this product were not evidence-based in orthopaedics. The marketing strategy employed by drug and implant companies trying to influence policy makers to get their products into the market when they cannot influence clinicians, was questioned.
Orthimo hips:
A query on Orthimo hips opened a debate that touched on various issues such as what could be classed as a new orthopaedic implant; governance issues behind introduction of new implants and industry-controlled price fixing of implants. There was a strong sensible view among vast majority of the members that new implants should be accepted only following a sound scientific scrutiny by engagement in programmes such as ‘Beyond Compliance’.
